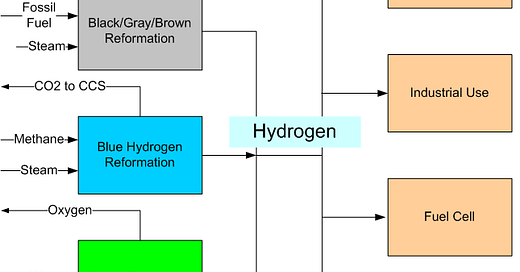
Manufacture and Use of Hydrogen
The above sketch provides an overview of how hydrogen is manufactured and used.
Manufacture of Hydrogen
The boxes on the left shows different types of hydrogen production. The boxes on the right show how the hydrogen is used.
At the top is the ‘Black/Gray/Brown’ section. This is how almost all hydrogen is produced now. A fossil fuel — generally methane — and steam are reacted with one another to form hydrogen and carbon dioxide (CO2). The CO2 is discharged to the atmosphere where it increases the concentration of greenhouse gases. Hydrogen as it is manufactured now is not a “green” fuel. The use of hydrogen made in the traditional manner increases the concentration of greenhouse gases in the atmosphere.
Next comes the ‘Blue’ section. Hydrogen is manufactured from methane and steam in the same way as for black, gray and brown hydrogen. However, the CO2 that is produced is captured and stored using Carbon Capture and Sequestration technology.
The third production box shows the ‘Green’ hydrogen production process. Water is electrolyzed to create hydrogen and oxygen. The oxygen can be sold commercially. This process does not add CO2 to the atmosphere (depending on the source of electricity that is used).
Users of Hydrogen
On the right side of the sketch are four types of user.
The first user is ‘Ammonia’. Although hydrogen has a high energy content, it is difficult to handle. One option is to convert it to ammonia, which is easier to use and which can be used as a fuel.
Next come the industrial users. They purchase the hydrogen for use in a wide range of facilities, including oil refineries.
Then there are fuel cells. The hydrogen is converted to electricity. This is the technology that is used in hydrogen-powered automobiles.
Finally, there is combustion. The hydrogen is burned directly. Its exhaust is water vapor, which is benign.