Hydrogen Safety: An Introduction
Hydrogen is often proposed as being a ‘green’ source of energy that can replace fossil fuels, particularly natural gas. Although the idea of using hydrogen as an energy source is attractive, there are many concerns to do with engineering feasibility, economics, and safety.
Our most recent post ― Process Safety Beacon: Hydrogen Leak ― described a hydrogen explosion that occurred in 2019 in California. In this post, we summarize some of the safety concerns to do with the use of hydrogen. This list is not complete ― future posts will provide further discussion on the topic of hydrogen safety.
1. Containment Challenges
Hydrogen, consisting of just two hydrogen atoms (H₂), is the smallest molecule. Its small molecular size and low viscosity allow it to leak through microscopic openings, seals, and fittings that would contain other gases. Even minute imperfections in welds or gaskets can result in measurable leakage. Therefore,
Hydrogen systems must use high-integrity seals and fittings specifically rated for hydrogen service.
Leak detection is essential. Specialized hydrogen sensors are often required, as traditional gas detectors may be ineffective.
Regular inspection and maintenance of piping, valves, and storage vessels is critical.
2. High Flammability and Auto-ignition
Hydrogen has an extremely wide flammable range in air: from 4% to 75% by volume, and it requires very little energy to ignite. Its minimum ignition energy is an order of magnitude lower than that of natural gas, meaning that even static electricity or small sparks can trigger combustion.
Moreover, hydrogen can auto-ignite under certain conditions, particularly when escaping from high-pressure systems where adiabatic compression or friction can generate sufficient heat for ignition. Therefore,
Venting and purging operations require careful control to prevent the formation of explosive mixtures.
All electrical and mechanical systems must be designed to prevent spark formation. The use of explosion-proof equipment, and proper grounding is important.
Pressure relief devices should discharge to safe, well-ventilated locations.
3. Invisible Flames
Hydrogen burns with a nearly invisible, pale blue flame that emits very little radiant heat. Personnel may be unaware that a fire is present until physical damage or secondary ignition occurs. Therefore,
Hydrogen facilities should use ultraviolet or infrared flame detectors capable of sensing hydrogen combustion.
Workers must be trained to recognize signs of hydrogen fires such as heat shimmer, noise, or the smell of burning materials, rather than the flame itself.
Fire protection systems must account for the non-luminous flame and the limited radiant heat.
4. Low Energy Density and High Pressure
While hydrogen has a high energy content per unit mass, its volumetric energy density is very low. To store useful amounts, it must be either compressed (often above 350–700 bar) or liquefied at cryogenic temperatures (–253°C). Therefore,
High-pressure systems increase stored energy and risk of rupture.
Cryogenic hydrogen presents additional hazards such as material embrittlement and frostbite.
Material compatibility and pressure relief design are essential safety considerations.
5. Embrittlement and Material Compatibility
Hydrogen can diffuse into metals and cause embrittlement, leading to cracking or sudden failure, particularly in high-strength steels. It is important to use materials certified for hydrogen service (e.g., austenitic stainless steels, aluminum
6. Ventilation and Dispersion Behavior
Hydrogen is the lightest gas known and disperses rapidly in open air, which can reduce hazard duration. However, in enclosed or semi-enclosed areas, it can accumulate near ceilings or under roof structures. Therefore, facilities should incorporate high-level ventilation and leak detection systems. Roof vents can help prevent gas buildup.
7. Emergency Response Considerations
Responding to hydrogen incidents requires special training and equipment.
Firefighters must use detectors to locate flames and avoid unnecessary risk.
Standard foam agents are often ineffective; dry chemical or water sprays may be used for cooling and control.
Evacuation zones should account for high flame speed and blast potential.
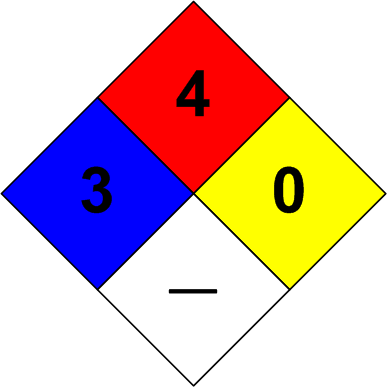
The Hydrogen Safety Diamond
The image at the head of this post is of the NFPA 704 ‘diamond’ for hydrogen. The colors have the following meanings.
Red ― Flammability
4: Severe hazard
Hydrogen ignites extremely easily.
Yellow ― Reactivity/Instability
0: Stable
Hydrogen is chemically stable under normal conditions. It doesn’t decompose or polymerize spontaneously. However, it reacts explosively with oxidizers (flammability).
White ― Special Hazards
None
If stored as liquid hydrogen (cryogenic), the white field may note “CRYO” or “LIQ” to indicate extreme cold.
Blue ― Health
3: Serious Hazard
Hydrogen is asphyxiating in confined spaces and can cause frostbite if released as a cryogenic liquid. It’s not toxic, but displacement of oxygen and rapid freezing injuries make it dangerous to personnel.