Summary
When there seems to be no solution to a problem then maybe we need to reframe the problem. Maybe we are asking the wrong question. For example, there seems to be no solution to the “dirty coal problem”. Here is the dilemma:
We need to stop adding carbon dioxide (CO2) to the atmosphere.
The fuel which adds the most CO2 to the atmosphere per unit of energy generated is coal. But, at a time of shortages of oil and natural gas many nations are ramping up their use of coal, regardless of its environmental impact. Indeed, a rapid phase out of coal would leak to severe economic setbacks.
It may be that this dilemma can be at least partially resolved by understanding that,
Coal is not the problem — nitrogen is the problem.
In this post we consider why coal is perceived as being dirty, and whether there are any technologies that will allow for the continued use of coal without emitting greenhouse gases.
“Dirty” Coal
Coal has a bad environmental reputation for two reasons.
It creates ash that has to be disposed of. This can be a serious problem if the ash contains potentially toxic compounds.
Coal contains a higher carbon/hydrogen ratio than natural gas. Hence, it produces more carbon dioxide (CO2) per unit of energy delivered than does natural gas.
Conventional Process
Figure 1 shows a schematic for the combustion of any hydrocarbon fuel in a normal boiler. The fuel (coal, oil or gas) contains carbon (C) and hydrogen (H). Both of these elements will burn and generate heat.
The air is a mixture of nitrogen (N2) and oxygen (O2) in an approximate ratio of 4 to 1. When the fuel is burned it creates carbon dioxide (CO2) — a greenhouse gas. The hydrogen creates water (H2O) vapor. The steam that is created in the boiler is normally used to spin a turbine which creates electricity that is delivered to the customers.
Note that the flue gas consists mostly of nitrogen, which has taken a “free ride” through the whole process. This fact that rarely receives much discussion.
Figure 1
Conventional Combustion
When the fuel is natural gas the CO2 concentration in the flue gas is about 5%. When the fuel is coal the CO2 concentration is about 8%. That’s not a big difference.
It is this small difference between 5% and 8% that makes coal “dirty”, but natural gas “clean”.
Replacing coal with natural gas is helpful, but it does not really address the core problem, which is how to capture the carbon dioxide generated by the burning of any fossil fuel.
Carbon Capture and Sequestration
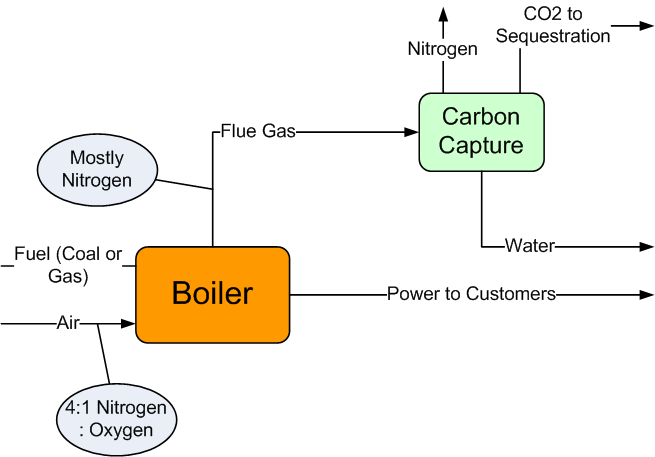
Carbon Capture & Sequestration (CC&S) technology removes CO2 from the flue gas, then compresses that CO2 and sequesters it underground, as illustrated in Figure 2. The nitrogen and condensed water vapor are disposed of safely.
Figure 2
Conventional Combustion with CC&S
CC&S technology is still in its commercial infancy. It does work, but the technology is expensive. Hence, there are very few operating units. One reason for its expense is that the concentration of CO2 in the flue gas is so low. We have seen that it is in the 5 to 8% range, regardless of whether the fuel is coal or natural gas. The remainder of the flue gas is mostly nitrogen. All that nitrogen takes up a lot of space, so the equipment has to be large (and expensive). Moreover, the nitrogen has to be heated and cooled; this is a cost with no benefit. In other words, the fundamental problem is not to do with carbon dioxide — it is to do with nitrogen.
Maybe the problem is not “dirty” coal — maybe the real problem is nitrogen.
If this insight is correct, then we need a technology that allows us to burn the fossil fuel (either coal or gas, it doesn’t matter which) while substantially reducing carbon capture costs. In other words, we need to stop adding nitrogen to the system. In other words, we need to look at Oxy-Combustion.
Oxy-Combustion
If we can remove the nitrogen from the combustion process then the flue gas will consist only of CO2, water vapor and small amounts of other compounds. The water vapor can be condensed and disposed of safely. The CO2 can be sequestered underground without any further treatment. There is no need for a Carbon Capture facility. The process for doing this is illustrated in Figure 3.
Figure 3
Oxy-Combustion
Air is fed to an Air Separation Unit (ASU). This is a technology that is very well understood and that is already very widely used in many commercial processes. The nitrogen from the ASU can be safely discarded into the atmosphere, or sold as a commercial product.
The oxygen is mixed with the hydrocarbon fuel (coal, oil or gas). The resulting flue gas consists just of carbon dioxide (CO2) and water vapor. The gas is passed through a condenser/cooler. The water effluent is treated and discarded; the CO2 is sequestered underground. (Some of the CO2 is compressed and recycled in order to maintain controlled combustion.)
There is no need for the carbon capture part of CC&S.
Conclusion
The point of this example is to show that we may be able to find effective responses to the climate crisis by reframing the problems that we face. In this example, both of the new processes are more expensive than our current mode of operation. But we cannot continue our current mode of operation — we have to accept that climate control comes at a cost. However, by reframing the problem, we may be able to find ways of using “dirty coal” while minimizing greenhouse gas emissions.