'Dirty' Coal
The phrase ‘dirty coal’ seems to have become one word. But all fossil fuels emit carbon dioxide (CO2) and other greenhouse gases. So why does coal have such a bad reputation?
There are two possible explanations. The first is that coal, being a solid, it leaves behind ash and slag that has to be disposed of. This is an environmental challenge, but it is a manageable challenge. The second reason for coal’s bad reputation is that it contains a higher carbon to hydrogen ratio than other fuels, particularly natural gas — which is primarily methane (CH4 — four hydrogen atoms for each carbon atom). Therefore, for a given energy output, coal emits more of the greenhouse gas carbon dioxide (CO2) than other fuels.
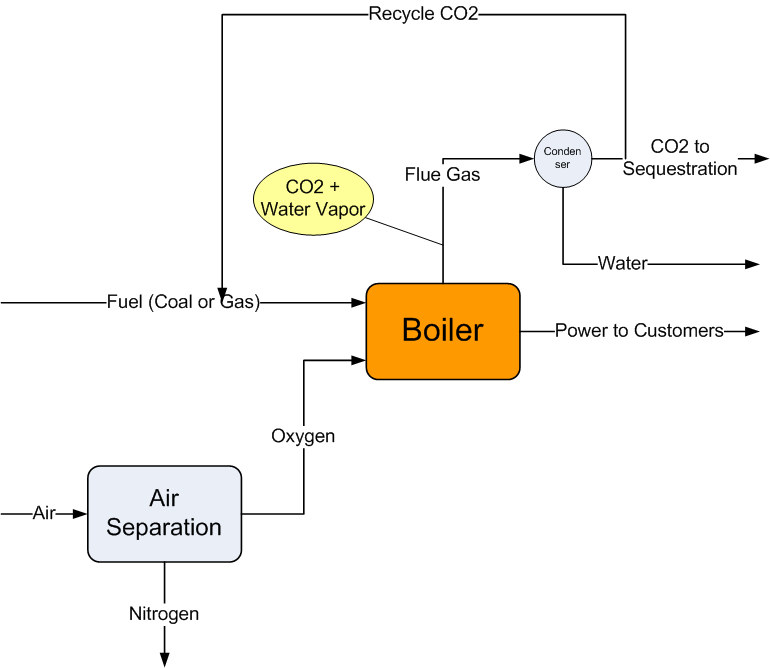
One way around this problem is to burn coal in an oxy-combustion process. Because nitrogen is removed from the air in the combustion gas the flue gas contains just CO2 and water vapor. The CO2 still has to be captured and sequestered. But, once the water content is condensed, the flue gas is almost pure CO2. Hence it is much easier and more economical to dispose of the CO2 compared to the carbon capture and sequestration technology needed for a conventional flue stack.
The following sketch illustrates how this process would work.
Keep reading with a 7-day free trial
Subscribe to Net Zero by 2050 to keep reading this post and get 7 days of free access to the full post archives.